Glycobiomarkers/in vitro diagnostics (IVDs), developed from innovative technologies in glyco science and glycomedicine, will contribute to human health.

Glycomedicine
Glycans or glycosylations are known to be modification of biomolecules, including lipids, proteins, and low- or high-molecular chemicals. Glycosylations are regulated by glycosyltransferases and their substrates and acceptors, which are affected by cellular conditions, such as differentiation, development, ageing, diseases and individual genetic background. Thus, glycans have been considered as good indicators, “glycobiomarkers”.
In the Glycoscience, new technologies based on engineering in addition to biochemistry and molecular biology promote new findings in medicine. For instances, changes of glycan structures of proteins are common features of cancer cells, associated with carcinogenesis, invasion and metastasis. “Glycomics and Glycoproteomics”, the research of glycans and glycproteins, are emerging fields in the postgenome and postproteomics era.
Hepatitis B Virus
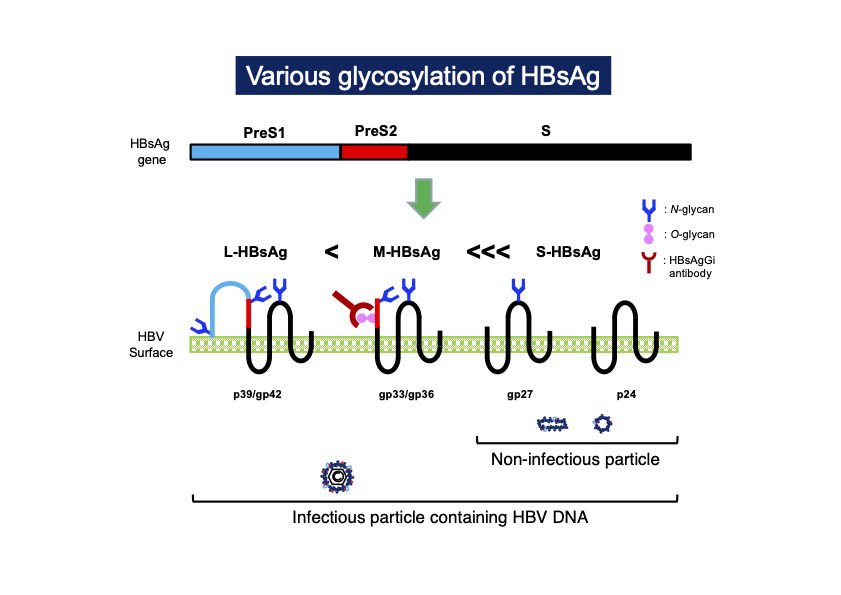
Hepatitis B virus (HBV), one of the most human infected viruses, has been a global health problem1. The HBV envelope protein is composed of three type of surface antigens, S-, M-, and L-HBsAg, which are produced from an HBsAg gene containing PreS1, PreS2, and S-domains2. HBsAgs are modulated by the chain of saccharides, such as N-glycan and O-glycan, which are involved in secretion and stability of the virus3,4. Recent glycotechnology revealed the difference of glycosylation in each HBsAg. PreS2 domain is present in M-HBsAg and L- HBsAg, the former contains O-glycan in PreS2 but the latter does not, in genotype C5,6. A recombinant monoclonal antibody against HBsAg glycan isomer (HBsAgGi) was cloned and available from RCMG Inc.
Technologies used in HBsAgGi development
- Glycome analysis to identify the structure of glycans
- Synthesis of glycosylated peptide
- Generating glycosylated HBsAg proteins
- Generating recombinant monoclonal antibody
Our targets in HBV
Infectious HBV particles (Dane particles) containing HBV DNA are less abundant than
subviral particles (SVP) in HBV patients, i.e. Dane particle <<< SVP. To monitor the presence of infectious HBV, technology measuring only Dane particle would be necessary rather than measuring whole HBV particles containing non-infectious SVP. Currently, nucleos(t)ide analogs (NA) treatment has been approved for the patients with chronic hepatitis B. NA treatment reduces HBV DNA, resulting in HBV RNA containing HBV particles, which might be infectious7,. Thus, convenient measuring method for HBV containing HBV RNA will be useful. Since HBsAgGi recognizes PreS2 domain in M-HBsAg of genotype C, following are current targets using HBsAgGi.
IVD by using HBsAgGi
- Measuring HBV DNA-containing HBV particles
- Capturing HBV particles to measure HBV DNA/RNA
- Measuring HBV RNA-containing HBV particles under NA treatment
- Measuring occult HBV with mutations in S-domain
- Correlation with cancer
References:
1. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
2. Schadler and Hildt (2009) Viruses 1:185-209. doi: 10.3390/v1020185.
3. Schmitt et al. (2004) J Gen Virol 85:2045-2053. doi: 10.1099/vir.0.79932-0.
4. Dobrica et al. (2020) Cells 9:1404. doi: 10.3390/cells9061404.
5. Wagatsuma et al. (2018) Anal Chem 90:10196-10203. doi: 10.1021/acs.analchem.8b01030
6. Angata et al. (2021) Biochim Biophys Acta Gen Subj. In Press. doi:10.1016/j.bbagen.2021.130020
7. Wang et al. (2016) J Hepatol. 65:700-710. doi: 10.1016/j.jhep.2016.05.029.